Benzinga - by Zacks, Benzinga Contributor.
Moderna, Inc. (NASDAQ: MRNA) announced that it has submitted an application to the FDA seeking approval of its Spikevax 2024-2025 formula, targeting the COVID-19 variant JN.1, the latest variant of the novel virus responsible for the 2020 pandemic.
The regulatory filing is based on guidance from the FDA last week, whereby the regulatory body advised eminent vaccine manufacturers like Moderna, Novavax (NASDAQ: NVAX) and Pfizer (NYSE: PFE) to update their respective COVID-19 vaccines to a monovalent (single strain) JN.1 composition for the 2024-2025 season.
The FDA's guidance was supported by the present study data that implies currently available COVID-19 vaccines are less effective against the variants in circulation. The Vaccines and Related Biological Products Advisory Committee (VRBPAC) of the FDA also unanimously voted in a meeting held on Jun 5 to recommend a monovalent JN.1-lineage vaccine composition.This was also recommended by a World Health Organization (WHO) advisory committeeand the European Medicines Agency's Emergency Task Force.
Please note that post the WHO recommendation, other subvariants like KP.3 and KP.2 have become the dominant strains in the United States since early June. Per the agency, these new strains are JN.1-derived subvariants that contain new additional mutations. Per the agency, these subvariants provide an advantage to the virus ‘either in terms of fitness or escape from immunity.'
Of the new mutations, the VRBPAC had expressed a strong preference for vaccines to be updated with JN.1 in the Jun 5 meeting. The FDA believes that a JN.1-variant targeting vaccine could also be effective against the other mutated strains. However, what is more concerning is that the virus' continuous evolution could lead to further divergence from the JN.1 variant.
The latest CDC data (as of Jun 8) shows that the KP.3 strain was the most prevalent, accounting for 25% of COVID-19 cases in the United States. It was followed by 22.5% and 14.9% for the KP.2 and LB.1 subvariants, respectively.
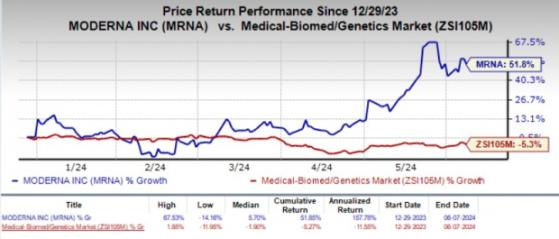
Year to date, shares of Moderna have rallied 51.8% against the industry's 5.3% decline.
Image Source: Zacks Investment Research
Moderna is simultaneously submitting regulatory applications worldwide to support registration and supply of the 2024-2025 formula of Spikevax in time for the upcoming vaccination season. Having already submitted an application for its Spikevax 2024-2025 formula in the United States, the company is expected to enjoy the ‘first mover's advantage' subject to approval.
Last week, Novavax stated that it plans to be ready for the 2024-2025 vaccination season with a JN.1 updated protein-based COVID-19 vaccine. The company is currently on track to deliver the recommended updated vaccines by September 2024, pending authorization. NVAX has been developing and manufacturing this vaccine at risk to ensure an adequate supply of vaccine shots ahead of the upcoming vaccination season.
Compared with Pfizer and Moderna, Novavax was not able to reap the benefits of the pandemic due to a delayed launch of its COVID-19 vaccine. During last year's vaccination season, sales of Novavax also suffered due to delayed approval of its vaccine formulation and product launch. Some investors and analysts believe that Novavax's early participation in the upcoming vaccination season could capitalize on the opportunity.
Moderna, Inc. Price and Consensus
Moderna, Inc. price-consensus-chart | Moderna, Inc. Quote
However, a variation in vaccine strain from the WHO-recommended strain could also pose a challenge for COVID-19 vaccine makers, especially Novavax, as it makes a more traditional protein-based shot that takes longer to manufacture. On the other hand, Pfizer and Moderna's vaccines, which are based on mRNA technology, can be developed more quickly when compared with Novavax's protein-based COVID-19 vaccine.
The vaccine makers are suffering a heavy beatdown in product sales and market value as COVID-19 cases have significantly dropped compared with the last couple of years. Though the rising COVID-19 infection cases could somewhat revive demand for vaccine boosters, players in this market are unlikely to witness the substantial gain in revenues and profits that was observed at the peak of the pandemic.
Zacks Rank and Stock to Consider Moderna currently carries a Zacks Rank #3 (Hold).
A better-ranked stock from the drug/biotech industry is ALX Oncology Holdings (NASDAQ: ALXO), carrying a Zacks Rank #2 (Buy) at present.
In the past 30 days, the Zacks Consensus Estimate for ALX Oncology's 2024 loss per share has narrowed from $3.33 to $2.89. During the same period, the consensus estimate for 2025 loss per share has narrowed from $2.85 to $2.73. Year to date, shares of ALXO have plunged 39.5%.
ALX Oncology beat estimates in two of the trailing four quarters and missed twice, delivering an average negative surprise of 8.83%.
To read this article on Zacks.com click here.
Read the original article on Benzinga