Pressing on with SDC-1801 in the clinic
Since initiating the three-part Phase Ia study in heathy volunteers (n=96) in May 2023, Sareum has completed part 1 (single ascending dose) and part 3 (food effects) of the study, with initial data indicative of a good safety profile and supporting once-daily oral dosing. The multiple ascending dose arm (part 2) is expected to conclude in Q2 CY24, by which time the company plans to announce full safety data from the Phase Ia study. Should data continue to be favourable and contingent on Sareum securing further funding (we expect the company is likely to seek non-dilutive funding in the form of a partnership), the company expects to start a Phase IIa study in psoriasis patients (n=24) before end-CY24.
Tightening the belt on costs
Given downward stock price pressure, Sareum has been unable to make drawdowns from the remaining c £2.5m of its RiverFort facility and it enacted stringent cost cutting measures to preserve cash. Sareum announced a £2.3m (gross) conditional equity raise, issuing 23m shares at 10p each (c 30% discount to 27 March closing). While dilutive, we see the raise as being necessitated by the company’s focus on timely completion of the Phase Ia study. At the H124 cash burn rate of c £1.9m (excluding the £760k finance charge related to the RiverFort facility), the pro-forma funds (£0.4m gross cash at end-H124, £0.4m in R&D tax credit received in Q1 CY24 and the £2.3m equity raise) would be projected to support runway extension into H2 CY24, past the Phase Ia study completion. Should data be positive, we expect the company to seek additional funds and/or partnering opportunities to undertake further clinical development for SDC-1801.
Approaching a key inflection point
TYK2/JAK1, a promising target in inflammatory conditions
SDC-1801, Sareum’s lead asset, is an inhibitor of both tyrosine kinase 2 (TYK2) and Janus kinase 1 (JAK1) enzymes, both part of the JAK family of proteins (four isoforms – JAK1, JAK2, JAK3 and TYK2) recognised for their role in immune regulation. These isoforms are known for their role in facilitating downstream signalling of a number of extracellular, pro-inflammatory cytokines, which lack their own enzymatic activity (Exhibit 1).
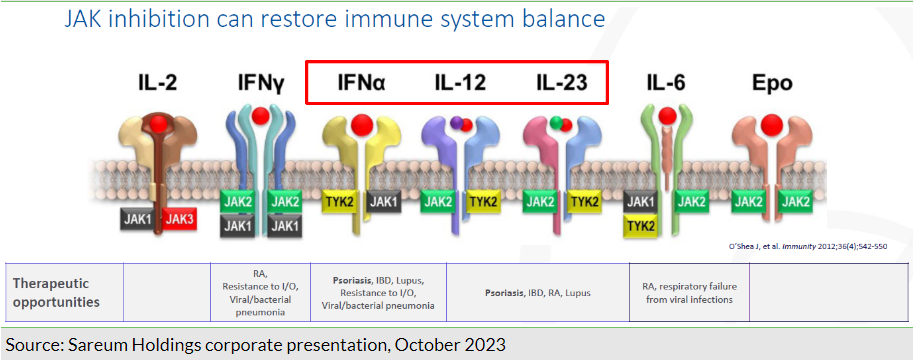
An oral administration targeting multiple cytokine pathways could offer theoretical advantages over single target agents such as biologics. TYK2, for example is instrumental in mediating the downstream signalling of IL-6, IL-10, IL-12, IL-23 and type I interferons (IFNs), recognised for their role in driving autoimmunity and associated inflammation. We also believe that a dual kinase approach, such as that of SDC-1801, accords applicability in a broad range of potential indications, particularly in the autoimmune space.
While effective, a non-selective and broad inhibition of certain JAK enzymes (particularly JAK2 and JAK3) has been associated with off-target toxicities, highlighted by the black box warnings (increased risk of malignancy, thrombosis and cardiac events) associated with the approved JAK1 inhibitors, Xeljanz, Olumiant and Rinvoq. SDC-1801 has been engineered to selectively target the TYK2/JAK1 enzymes with the aim of circumventing the safety/toxicity issues related to the JAK2 and JAK3 isoforms. Although SDC-1801 is designed to target a range of autoimmune diseases, the initial focus is on psoriasis, a serious dermatological condition affecting more than 125 million people globally (7.5–8.0 million in the US alone). The global psoriasis treatment market was valued by Fortune Business Insights at US$26.4bn in 2022 and is expected to grow to US$51.5bn by 2030.
The psoriasis treatment market has hitherto been dominated by biologics – anti-TNF and interleukin antibodies – although the approval of Bristol Myers Squibb’s first-in-class, selective TYK2 asset, Sotyktu (deucravacitinib) in September 2022 could potentially start to change that. Sotyktu was approved without a black box warning and without restricted usage (such as limitations to patients refractory to biologics and other available treatments), demonstrating the potential of this class of drugs (Sotyktu recorded global sales of US$170m in 2023, its first full year on the market). Sareum claims that the dual TYK2 and JAK 1 inhibition offered by SDC-1801 could result in better efficacy (without safety trade-offs) in psoriasis compared to agents that target only one of the two kinases, although this would have to be proved in large clinical trials.
SDC-1801 moving steadily through the clinic
Sareum initiated clinical studies for SDC-1801 in Australia in June 2023 (Phase Ia), following approval of its clinical trial application by the Australian authorities in May 2023. The Phase Ia study is a randomised, placebo-controlled trial (targeted n=96) evaluating safety, tolerability and pharmacokinetics/pharmacodynamics of SDC-1801 in healthy adults. The study includes three parts: a single ascending dose (SAD) study (part 1), a multiple ascending dose (MAD) study (part 2) and a food effects study (part 3). In February 2024, the company announced that part 1 and part 3 of the study have been completed, indicating a favourable safety profile and supporting once-daily oral dosing to achieve therapeutic drug levels in the bloodstream. The SAD part randomised participants in a 3:1 ratio to a single ascending dose xof SDC-1801 or placebo in six dose cohorts with eight subjects each. Part 2 (MAD) is ongoing (four sequential and ascending doses tested on four dose cohorts with eight participants each) and is expected to complete by Q2 CY24. Full safety data from the Phase Ia study is planned to be reported in Q2 CY24 and we believe this could be a key inflection point for the company. Sareum had previously planned to initiate the follow-up Phase IIa study (with 24 psoriasis patients) in H2 CY24 (with a planned completion by end-CY24) but given the funding constraint, the timeline for trial initiation has now been pushed out by a few months to end-CY24, subject to the Phase Ia data review and access to financing. If the Phase Ia data are positive, we believe Sareum will likely seek potential out-licensing opportunities, whereby a prospective partner would be responsible for undertaking the next phase of clinical development as well as subsequent commercialisation.
In March 2024, Sareum received a notice of allowance from the European Patent Office, related to SDC-1801’s (application (EP3864009) in the treatment of autoimmune diseases, and certain methods of synthesis. This follows the approval in China in June 2023 (CN113056456), which was the first patent granted to SDC-1801 and Japan. Patent applications in several other regions are under review, including the US (US2021387981).
A broader pipeline, beyond SDC-1801
While Sareum has other assets in its pipeline (including another TYK2/JAK1 inhibitor, SDC-1802, targeting cancer immunotherapy), we understand that development work on these assets has been put on hold to focus resources on the clinical development of SDC-1801. We believe that development work may be reinitiated following a partnering deal for the lead asset.
SDC-1802: management has indicated that work on translational studies is ongoing to identify an optimal cancer indication and patient population before undertaking further toxicology and manufacturing studies. We remind readers that in June 2023, Sareum was granted a patent for SDC-1802 in the US for autoimmune disorders, extending its scope beyond the initial focus immuno-oncology. The first patent for the compound was granted in April 2022, covering the molecular and pharmaceutical preparations of SDC‐1802 in the treatment of T‐cell acute lymphoblastic leukaemia and other cancers.
SRA737: SRA737 is an oral checkpoint kinase 1 (CHK1) inhibitor, in which Sareum owns a 27.5% economic interest (the remaining stake is held by CRT Pioneer Fund). Rights to the drug (previously out-licensed to Sierra Oncology, which was subsequently acquired by GSK (LON:GSK)) were returned to the original owners (Sareum and CRT Pioneer Fund) in November 2022. In December 2023, CRT Pioneer Fund out-licensed SRA737 to a privately-held US biopharma for an upfront payment of US$0.5m, additional fees of up to $1m in cash and 500,000 shares of the partner and potential milestone payments of up to $289m. The deal also allows for tiered high single-digit royalties on net sales. As part of the deal, Sareum received $137.5k as an upfront payment and is also entitled to receive 27.5% of any future payments.
Financials
Widened operating loss on increased clinical activity
Sareum reported an H124 (six months ending December 2023) operating loss of £2.53m, up from £1.75m in H123, driven by increased investments in preparatory activities related to the Phase Ia clinical trial of SDC-1801. The H124 net loss was £2.51m, with the R&D tax credit of £0.77m received during the period offset by the £0.76m finance charge resulting from the £5m RiverFort prepayment facility entered into on 3 August 2023 (£2.3m drawn down to date in two tranches). This finance charge reflects the difference between the amount due to RiverFort as at 31 December 2023 in respect of shares issued to it, and the market value of the shares it still held at that date. Reflecting the higher operating expenses during the period, the net cash outflow from operating activities increased to £2.69m in H124 from £1.34m in H123.
The economics of the deal with RiverFort included Sareum issuing shares to cover the drawdown amount, which RiverFort would subsequently sell in the market. With the recent downward pressure on Sareum’s share price (59.5p at 31 December 2023 vs 100p at the time of deal signing), shares sold (worth £0.7m) and held (worth £0.5m) by RiverFort fall short of covering the loaned amount, resulting in the non-case finance charge of £0.76m in Sareum’s books. Note that this charge is subject to change with the movement in Sareum’s share price and the sale of further shares by RiverFort. Exhibit 2 presents a snapshot of the drawdowns/conversions to date and the outstanding balance.
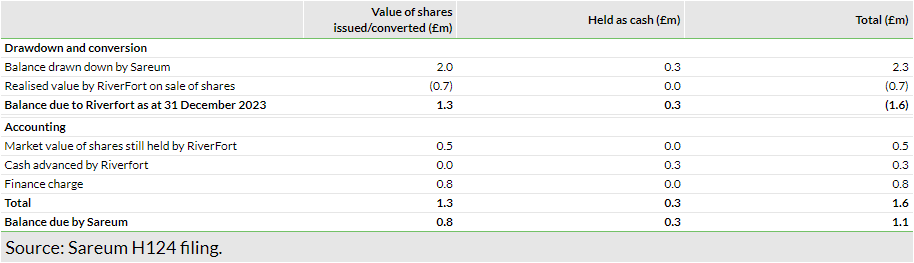
As of 26 March 2024, £1.1m remains outstanding for repayment under the facility, which Sareum expects to fulfil through share issuances (or cash payment) on or before August 2025. With the SDC-1801 clinical trial in full force, operating expenditure rates may remain at comparable levels (vs H124) in H224, although we note that the company may generate some savings following its decision to minimise cash utilisation (as highlighted by its decision to settle pending salaries of directors/advisors in shares and deferring a portion of their salaries to a future date).
New funds extend runway
The gross cash balance at end H124 stood at £0.4m versus £1.0m at end FY23 and £2.9m at end H123. This was further supported by the £0.4m tax credit received in January 2024 and the £2.3m conditional equity fund raise (before expenses) announced by the company on 28 March 2024 and subsequently updated on 2 April 2024. This fund raise would result in an issuance of 23.2 million new ordinary shares at the placing price of 10p/share (a ~30% discount to the closing price of 14.5p on 27 March). This includes 9.55m shares in an open placement (‘placing’), 2.255m shares under a direct issue to certain high net worth individuals (‘subscription’), 195k shares to company directors and 11.2m shares in a retail offering (WRAP retail offer) to existing shareholders. The WRAP retail offer closed on 2 April 2024, and new shares are expected to be admitted for trading on AIM on 5 April 2024.
The completion of the placing is conditional, among other things, on the company receiving a commitment of a minimum of £0.205m under the subscription prior to 4 April 2024 and on admission of the new ordinary shares for trading on the AIM exchange. We believe that the need to raise external equity capital was triggered by the company’s inability to make further drawdowns from the £5m prepayment facility with RiverFort.
Management estimates that, along with cash at hand, the tax credits of £0.4m received in January 2024 and the net proceeds from the fundraise will be sufficient to complete the Phase Ia clinical trial of SDC-1801, and it expects to report top-line safety data from the trial in Q2 CY24. Furthermore, the company anticipates receiving tax credits of £0.7m in September 2024, which would support the company in meeting working capital needs. At the company’s current run-rate (cash burn of £1.9m in H124, excluding the £0.76m finance charge), it may need to secure additional funds in H2 CY24 to advance SDC-1801 to the Phase IIa trial.
_________________________________________
General disclaimer and copyright
This report has been commissioned by Sareum Holdings and prepared and issued by Edison, in consideration of a fee payable by Sareum Holdings. Edison Investment Research standard fees are £60,000 pa for the production and broad dissemination of a detailed note (Outlook) following by regular (typically quarterly) update notes. Fees are paid upfront in cash without recourse. Edison may seek additional fees for the provision of roadshows and related IR services for the client but does not get remunerated for any investment banking services. We never take payment in stock, options or warrants for any of our services.
Accuracy of content: All information used in the publication of this report has been compiled from publicly available sources that are believed to be reliable, however we do not guarantee the accuracy or completeness of this report and have not sought for this information to be independently verified. Opinions contained in this report represent those of the research department of Edison at the time of publication. Forward-looking information or statements in this report contain information that is based on assumptions, forecasts of future results, estimates of amounts not yet determinable, and therefore involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of their subject matter to be materially different from current expectations.
Exclusion of Liability: To the fullest extent allowed by law, Edison shall not be liable for any direct, indirect or consequential losses, loss of profits, damages, costs or expenses incurred or suffered by you arising out or in connection with the access to, use of or reliance on any information contained on this note.
No personalised advice: The information that we provide should not be construed in any manner whatsoever as, personalised advice. Also, the information provided by us should not be construed by any subscriber or prospective subscriber as Edison’s solicitation to effect, or attempt to effect, any transaction in a security. The securities described in the report may not be eligible for sale in all jurisdictions or to certain categories of investors.
Investment in securities mentioned: Edison has a restrictive policy relating to personal dealing and conflicts of interest. Edison Group does not conduct any investment business and, accordingly, does not itself hold any positions in the securities mentioned in this report. However, the respective directors, officers, employees and contractors of Edison may have a position in any or related securities mentioned in this report, subject to Edison's policies on personal dealing and conflicts of interest.
Copyright: Copyright 2024 Edison Investment Research Limited (Edison).
Australia
Edison Investment Research Pty Ltd (Edison AU) is the Australian subsidiary of Edison. Edison AU is a Corporate Authorised Representative (1252501) of Crown Wealth Group Pty Ltd who holds an Australian Financial Services Licence (Number: 494274). This research is issued in Australia by Edison AU and any access to it, is intended only for "wholesale clients" within the meaning of the Corporations Act 2001 of Australia. Any advice given by Edison AU is general advice only and does not take into account your personal circumstances, needs or objectives. You should, before acting on this advice, consider the appropriateness of the advice, having regard to your objectives, financial situation and needs. If our advice relates to the acquisition, or possible acquisition, of a particular financial product you should read any relevant Product Disclosure Statement or like instrument.
New Zealand
The research in this document is intended for New Zealand resident professional financial advisers or brokers (for use in their roles as financial advisers or brokers) and habitual investors who are “wholesale clients” for the purpose of the Financial Advisers Act 2008 (FAA) (as described in sections 5(c) (1)(a), (b) and (c) of the FAA). This is not a solicitation or inducement to buy, sell, subscribe, or underwrite any securities mentioned or in the topic of this document. For the purpose of the FAA, the content of this report is of a general nature, is intended as a source of general information only and is not intended to constitute a recommendation or opinion in relation to acquiring or disposing (including refraining from acquiring or disposing) of securities. The distribution of this document is not a “personalised service” and, to the extent that it contains any financial advice, is intended only as a “class service” provided by Edison within the meaning of the FAA (i.e. without taking into account the particular financial situation or goals of any person). As such, it should not be relied upon in making an investment decision.
United Kingdom
This document is prepared and provided by Edison for information purposes only and should not be construed as an offer or solicitation for investment in any securities mentioned or in the topic of this document. A marketing communication under FCA Rules, this document has not been prepared in accordance with the legal requirements designed to promote the independence of investment research and is not subject to any prohibition on dealing ahead of the dissemination of investment research.
This Communication is being distributed in the United Kingdom and is directed only at (i) persons having professional experience in matters relating to investments, i.e. investment professionals within the meaning of Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the "FPO") (ii) high net-worth companies, unincorporated associations or other bodies within the meaning of Article 49 of the FPO and (iii) persons to whom it is otherwise lawful to distribute it. The investment or investment activity to which this document relates is available only to such persons. It is not intended that this document be distributed or passed on, directly or indirectly, to any other class of persons and in any event and under no circumstances should persons of any other description rely on or act upon the contents of this document.
This Communication is being supplied to you solely for your information and may not be reproduced by, further distributed to or published in whole or in part by, any other person.
United States
Edison relies upon the "publishers' exclusion" from the definition of investment adviser under Section 202(a)(11) of the Investment Advisers Act of 1940 and corresponding state securities laws. This report is a bona fide publication of general and regular circulation offering impersonal investment-related advice, not tailored to a specific investment portfolio or the needs of current and/or prospective subscribers. As such, Edison does not offer or provide personal advice and the research provided is for informational purposes only. No mention of a particular security in this report constitutes a recommendation to buy, sell or hold that or any security, or that any particular security, portfolio of securities, transaction or investment strategy is suitable for any specific person.
London │ New York │ Frankfurt
20 Red Lion Street
London, WC1R 4PS
United Kingdom
