In a positive development, Midatech (LON:MTPH) (NASDAQ:MTP) has announced the US FDA has granted fast-track designation for its lead clinical asset, MTX110 in recurrent glioblastoma (rGBM). This should allow to company to apply for a fast-track approval and a potentially faster market entry, provided supportive Phase II data are obtained. MTX110 is expected to start a Phase I study in (rGBM) in mid-2022 with early progression-free survival data expected by Q422. The stock closed up 24.4% following the announcement.
In our last note, we highlighted MTX110’s potential longer time to market for rGBM (versus diffuse intrinsic pontine glioma) as a partial offset to its materially larger market opportunity. This announcement largely allays those concerns while keeping the upside option intact. We also see partnership and licensing discussions picking momentum after this news, which could potentially crystallise into a deal on the back of positive proof of concept or Phase II read-outs. We emphasise again the aggressive nature of the cancer (average survival of 12–18 months with standard of care, three months without treatment) and the small number of therapeutic options. These are temozolomide (chemotherapy), bevacizumab (anti-VEGF monoclonal antibody; not approved in Europe) and nitrosourea/gliadel wafer (chemotherapy), which together make a sizeable $2–5bn market, according to management.
The drug under focus, MTX110, uses the company’s proprietary MidaSolve technology to solubilise the chemotherapy drug panobinostat, which is then delivered through a convection-enhanced delivery system directly to the site of the tumour. The planned Phase I study is expected to start by mid-2022 across two clinical centres in the United States (Duke University and MD Anderson Cancer Center). The primary objectives will be to assess safety and tolerability in patients with rGBM (dose-escalation study estimated to recruit between 10 and 12 patients), but the study will also track preliminary efficacy signals such as progression free survival. Midatech expects to release preliminary data from the study by Q422.
Business description
Midatech Pharma is platform-based drug delivery specialist founded in 2000 and listed on the AIM in 2014. Its three technology platforms, Q-Sphera (for sustained release of drugs), MidaSolve (nano inclusion for local delivery) and MidaCore (gold nanoparticles for targeted delivery), are designed to re-engineer and reformulate existing therapeutic drugs with the aim of improving biodistribution and delivery. The realigned focus is now on the Q-Sphera development pipeline and the clinical asset MTX110 (for brain cancer).
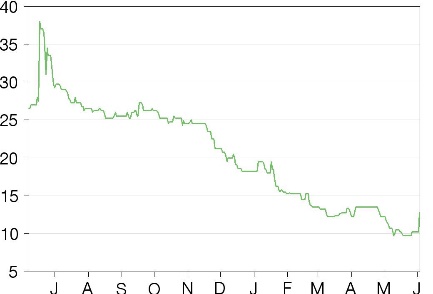
Share price performance