BioPorto has announced the appointment of Anthony Pare and Neil Goldman as its new CEO and CFO, respectively, with effect from mid-November 2021. In addition, Thomas Magnussen, chairman of the Board of Directors, has decided to step down from his position, with vice chairman Christopher Lindop taking over. We note that both the CEO and CFO are US-domiciled and view these appointments as strategic given the company’s increasing focus on the US market as it inches closer to the completion of clinical trials and a subsequent de novo filing for its NGAL Test in paediatric patients.
Share price performance
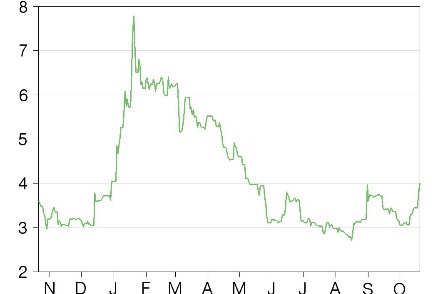
Business description
BioPorto Diagnostics is a diagnostic company focused on the development and commercialisation of biomarker-based assays. Its portfolio includes the NGAL Test, for prediction of acute kidney injury, and an extensive antibody library. The company is currently undertaking clinical trials in paediatric patients, which are expected to conclude by the end of 2021, followed by the 501(k) de novo FDA filing. Approval in paediatric patients would be followed by studies in the adult population.
Both the new CEO and CFO come with strong technical and leadership backgrounds, with decades of executive experience between them. Incoming CEO Anthony Pare previously served as chief commercial officer of T2 Biosystems, an in vitro diagnostics company, and CFO Neil Goldman joins from Chembio Diagnostics, a global developer of point-of-care tests for infectious disease, having served as its CFO. Both companies are NASDAQ-listed.
The new appointments come at a critical juncture for BioPorto, as it is at a vital transition phase. The new United States-based appointments confirm the company’s increasing focus on progressing the NGAL Test to commercialisation as it approaches clinical approval, with the US being the key market.
BioPorto is approaching a key inflection point in its path to commercialisation. By the end of 2021, it expects to complete the pivotal study assessing its NGAL diagnostic test in critically ill paediatric patients at risk of moderate to severe acute kidney injury (AKI). BioPorto recently completed interim analysis of data (from six US children’s hospitals over a 12-month period) from the paediatric clinical trials and, while the data have not been made public, the company states that the results (in terms of sensitivity and specificity) were encouraging and support further enrolment and conclusion of the study. BioPorto is currently expanding patient enrolment to maximise the study’s statistical power and intends to onboard a further three partner hospitals to its existing 12 sites. Following conclusion of the clinical study, the company plans to apply for regulatory submission to the FDA for de novo 510(k) clearance in early 2022. In our experience, approvals in this category typically take three to six months, which could point to FDA approval as soon as Q2/Q322. Based on the results of the paediatric application, BioPorto will decide on the roadmap for the clinical studies and subsequent approval for the adult NGAL Test, a meaningfully larger market for the company.
